Dr. Ruiz Introduces Bipartisan Legislation to Expand Seniors’ Access to Life-Saving Cancer Screenings
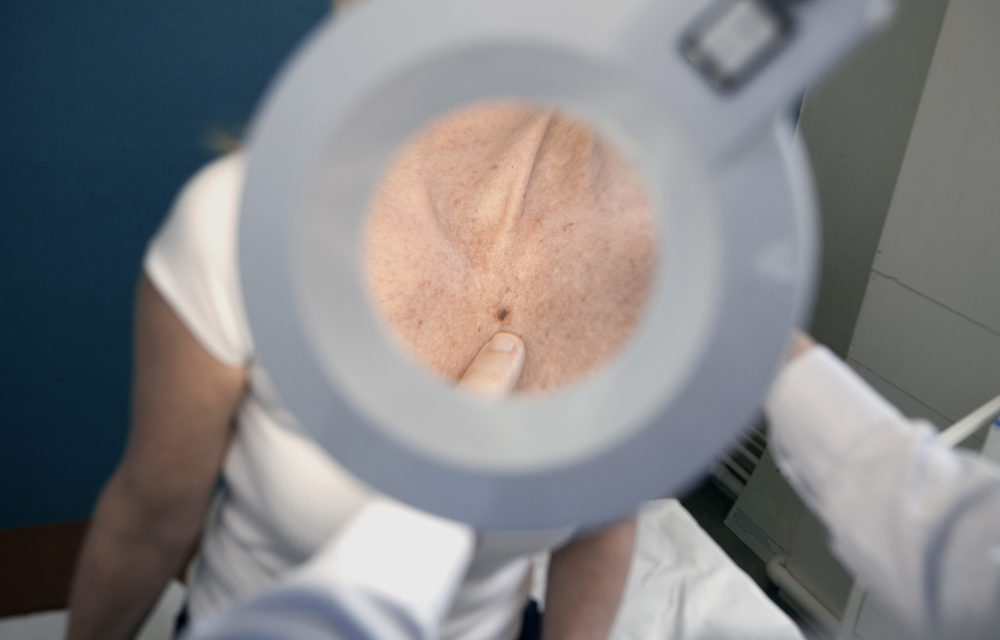
WASHINGTON, DC — Today, Congressman Raul Ruiz, M.D. , CA-36, introduced legislation to ensure timely Medicare coverage of groundbreaking early cancer screenings for the nation’s seniors, according to a news release.
The bipartisan legislation recognizes emerging advances in the nation’s fight against cancer by creating a Medicare coverage pathway for new innovative tests that detect multiple types of cancer before symptoms develop. Dr. Ruiz introduced the Multi-Cancer Early Detection Screening Coverage Act alongside Reps. Terri Sewell (AL-07), Jodey Arrington (TX-19), and Richard Hudson (NC-08).
“Detecting cancer early improves health outcomes and saves lives,” said Dr. Ruiz (CA-36). “With innovative medical technologies, we can catch more cancers earlier than ever, but patients must be able to access these screenings. That’s why it’s very important for Medicare policy to keep pace with the development of multi-cancer screenings. I am glad to join my colleagues in introducing the Multi-Cancer Early Detection Screening Coverage Act of 2020 to modernize Medicare coverage so older Americans can access these screenings and improve their health outcomes.”
The Multi-Cancer Early Detection Screening Coverage Act has the support of the National Minority Quality Forum (NMQF) and the American Cancer Society Cancer Action Network (ACS CAN).
The Multi-Cancer Early Detection Screening Coverage Act responds to advances in science and Medicare coverage by creating a new Medicare coverage pathway for multi-cancer screening. The legislation would:
- Create the authority for CMS to cover blood-based multi-cancer early detection tests and future test methods (like urine or hair tests) once approved by the Food and Drug Administration (FDA).
- Maintain CMS’ authority to use an evidence-based process to determine coverage parameters for these new tests.
- State that these new tools will complement – not replace – existing screenings and coverage and cost-sharing will not be impacted.
Under current law, Medicare coverage of preventive services is limited to circumstances in which Congress has explicitly authorized coverage or the U.S. Preventive Services Task Force recommends the service with a grade of A or B. In the absence of this legislation, it could take several years after the FDA approval before Medicare beneficiaries could receive coverage for such tests.
This bill would reduce any such access delays for seniors while allowing CMS to use its evidence-based process to determine coverage. Accordingly, these new multi-cancer screening tools will complement existing screenings and dramatically improve our nation’s cancer early detection capabilities.
“This year has exposed the inequities in our nation’s healthcare system, but we have known for some time that cancer has an outsized impact on communities of color,” Gary A. Puckrein, PhD, President and CEO of NMQF, said in a prepared statement. “We must be ready to take full advantage of new technologies that give us the chance to not only close the gap in cancer outcomes among racial and ethnic groups, but also reduce patient risk for everyone. New multi-cancer early detection tests hold that promise and this legislation is essential to helping us achieve it.”
“We know early detection of cancer saves lives. By creating a pathway for Medicare to cover new, innovative, FDA-approved screening tests, this legislation would help provide more individuals with access to additional tools they and their providers can utilize to detect cancer at an earlier, more treatable stage,” said Lisa Lacasse, president of the ACS CAN.
The text of the Multi-Cancer Early Detection Screening Coverage Act can be found here.
Image Sources
- Dermatology, Symptomatology Elderly Person: Shutterstock